Quality
Quality Contol & Assurance
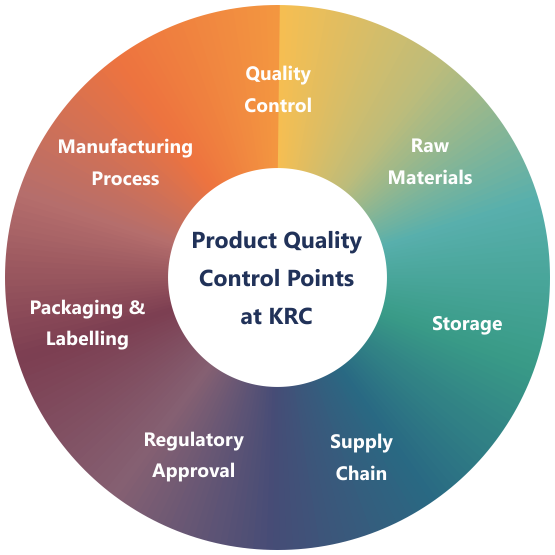
Testing of your raw materials and active ingredients ensures the quality of your products. At KRC, laboratory controls are an integral part of Quality Manufacturing Practices and the requirements thereof are described in the regulations. Quality controls for raw materials and ingredients comprise identity, purity and content testing and are carried out following the regulations of the Pharmacopoeia or according to the customer's own instructions.



Facilities for QC & QA
| Sr. No. | Instrument | Make | Remark |
|---|---|---|---|
| 1 | GC | Shimadzu | GC-2010 plus |
| 2 | HPLC | Shimadzu | LC-2010 plus |
| 3 | Karl Fisher | Veego | - |
| 4 | Melting Apparition | Veego | - |
| 5 | UV Chamber | - | - |
| 6 | pH Meter | Lab Man | Range -2 to 20 |
Commitment for Quality
KRC is committed to manufacture and supply of products of consistently high standard that meet customers' needs and fulfil their expectations. The highest priority is placed on the integrity of our products, their safe manufacture and our adherence to environmental and other international regulations.
KRC operates a process-oriented Quality Management System based on the international quality standard ISO 9001. Training plays a pivotal role in this system. Dedicated SOPs describe details of our training regime. Continuous improvement of our systems, products and services is a key focus of KRC. As a supplier to the pharmaceutical industry, KRC Chemicals is committed to current Good Manufacturing Practices (cGMP) procedures and ensure that our global manufacturing sites adhere fully to our internal GMP policies, local regulations and the international guidelines.
Quality Products by...
- Technical Expertise
- Quality Control
- Quality Assurance